【Carbon】From Carbon Products to the Development of the Carbon Industry

【Carbon】From Carbon Products to the Development of the Carbon Industry
Overview of Carbon Development
Human use of carbon has evolved alongside human development. In ancient times, humans began using carbon (charcoal) when they discovered and harnessed fire. In China, the Geography of the Former Han recorded, "Yuzhang Commandery produces stones that can burn as firewood," referring to coal; the Records of the Grand Historian – Biography of Empress Dowager Dou recorded coal mining, indicating that coal was already used as fuel at that time. Charcoal and coal were widely used during the eras of pottery and bronze. As carbon products, the Tiangong Kaiwu (1637) by Song Yingxing in the Ming Dynasty already recorded the use of carbon (graphite) and clay to make crucibles, representing the earliest form of carbon products.
Some sources divide the application of carbon into the following stages: the Charcoal Era (~1712), the Coal Era (1713–1866), the Cradle Era of Carbon Products (1867–1895), the Industrialization Era of Carbon Products (1896–1945), the Development Era of Carbon Products (1946–1985), and the New Carbon Era (1986–). This division roughly summarizes the development process of carbon, though the timing is not entirely precise. For example, coal was applied in China long before 1713, and graphite-clay crucibles had already been made.
In 1713, A. Derby invented the production of coke from coal and began using metallurgical coke to smelt iron, marking the true beginning of carbon as an industrial material. In 1800, British scientist H. Davy used charcoal slices as arc electrodes, later crushing charcoal, mixing it with coal tar, molding it, and baking it to form the earliest carbon electrodes. In 1842, R. Bunsen used charcoal and charred residues, calcined, crushed, and sieved, then mixed the carbon aggregate powder with a binder in proportion. The mixture was pressed in molds, covered with coke powder to isolate air, slowly heated for baking, and then mechanically processed to produce carbon electrodes, the prototype of modern carbon products. The conventional production process of carbon products today still retains this method. Subsequently, dense electrodes and natural graphite linings for blast furnaces were developed.
In 1867, W. Siemens invented the electric motor, marking the arrival of the electrical era and promoting the widespread development of carbon products such as arc carbon electrodes, steelmaking electrodes, electrolytic salt carbon anodes, and motor brushes. In 1886, P. Heroult and C. Hall invented aluminum electrolysis, using anode paste and carbon anodes in production. Subsequently, carbon anodes were also applied in the production of yellow phosphorus, ferroalloys, and calcium carbide (electric stone).
In 1896, E.G. Acheson developed artificial graphite electrodes, transforming carbon products from carbon-based to graphite-based, marking the entry of carbon products into the industrialization era. Subsequently, dense carbon brushes, graphite plates for electrolysis, and impermeable graphite products were successively developed. Particularly in 1942, E. Fermi produced high-purity nuclear graphite for nuclear reactors. After the 1940s, with the rapid development of industry and the continuous emergence of new technologies and processes, the production scale of carbon products expanded, and new applications appeared, driving the development of many new carbon materials and products.
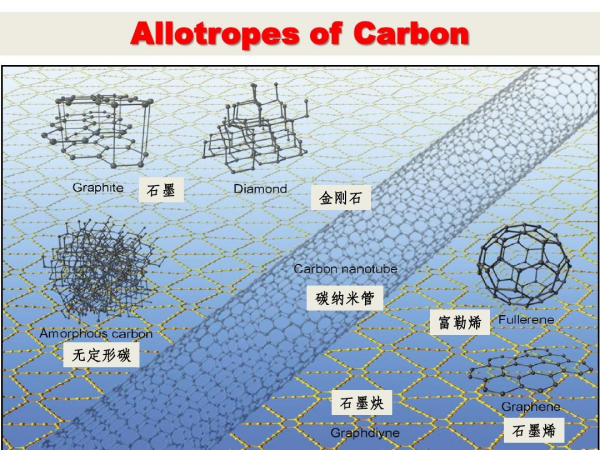
New carbon materials include carbon fibers and composites, nuclear graphite, pyrolytic graphite, glassy carbon, impermeable graphite, and porous carbon. New products include graphite molds for metallurgical machinery, carbon bricks for ironmaking, blast furnaces and other smelting furnaces, graphite heat exchangers, graphite bearings and seals, heating elements and boats for high-temperature furnaces, VHP electrodes, fuel cell bipolar plates, EDM electrodes, graphite rocket nose cones and throat liners. Particularly, the discovery of Mesophase Carbon microspheres, fullerene C60 (1985), carbon nanotubes (1991), and graphene (2004) ushered carbon materials into the New Carbon Era.
China's carbon industry started relatively late, gradually forming in the mid-1950s. Since the 1980s, the carbon industry has developed rapidly. Today, China's total output of carbon materials and products ranks first in the world, making China a major carbon nation. However, there is still a gap with advanced countries in high-end materials and products, especially carbon fiber and composites, which are limited in variety, performance, and high price, restricting their applications. China aspires to quickly become a world carbon superpower.
Differences and Similarities Between Carbon Materials and Carbon Products
Carbon products are a general term for carbonaceous and graphite products. There is currently no strict definition of "carbon material" or "carbon product." Broadly speaking, there is no major difference. For example, prebaked anodes can be called either anode materials or anode products. Upon closer examination, differences do exist. "Carbon material" primarily refers to the type of material and microstructure, generally indicating a raw carbon product that cannot yet be used directly and requires mechanical processing. "Carbon product" generally refers to a mechanically processed raw product that can be directly used with precise dimensions, surface finish, and tolerances. For example, isostatically pressed blanks can be called isostatic materials; if used to make heaters for monocrystalline silicon furnaces, insulation cylinders, graphite electrodes, or graphite supports, they are called carbon (or graphite) products, or graphite parts. A heater, for example, is strictly a "product" and not a "material," or it can only be described as being made from isostatic graphite material.
Classification of Carbon Products
There is no unified standard or strict principle for classifying carbon (graphite) products. Generally, they can be classified as follows:
By material or microstructure:
1) Carbon products: material is amorphous carbon (carbonaceous);
2) Graphite products: material is graphite (graphitic);
3) Diamond products: material is diamond (not discussed in this book; only the first two types are covered).
By aggregate particle size:
1) Coarse-grained carbon products: maximum aggregate size ≥ 1 mm (16 mesh);
2) Fine-grained carbon products: maximum aggregate size 1–0.25 mm (16–60 mesh);
3) Fine-structure carbon products: maximum aggregate size 0.25–0.038 mm (60–400 mesh);
4) Ultrafine powder carbon products: powder size <38 μm;
5) Nano carbon products: powder size in tens of nanometers or smaller.
By purity:
1) High-ash products: ash content >1%;
2) Low-ash products: ash content 1–0.1%;
3) Low-purity products: ash content 0.1–0.01%;
4) High-purity products: ash <0.01%;
5) Ultra-high-purity products: ash <50 mg/L.
By production process:
1) Conventional products;
2) Fullerene, carbon nanotube, and graphene products;
3) Carbon fiber and composite products;
4) Synthetic diamond and superhard materials;
5) Pyrolytic carbon (graphite) products;
6) Glassy carbon products;
7) Activated carbon products;
8) Carbon black products.
Additionally, there are porous carbon, mesophase carbon microspheres, surface coatings, flexible graphite, colloidal graphite, etc.
By function and application:
1) Aerospace and military carbon products;
2) Nuclear graphite products;
3) Electronic, electrical, and communications carbon products;
4) Mechanical carbon products;
5) Electrochemical carbon products;
6) Chemical impermeable carbon/graphite products;
7) Electrothermal, metallurgical carbon products;
8) Industrial furnace carbon/graphite products;
9) Measurement and metrology carbon products;
10) Environmental, sports, and daily-use carbon products.
These categories can be further subdivided. Other classification methods exist but are not listed here.

The Role and Position of the Carbon Industry and Its Products in National Economic Development
Compared with large industries such as machinery, automotive, electronics, mining, metallurgy, railways, and transportation, the carbon industry is small but occupies an important position in the national economy and plays a significant role. For example, in China's First Five-Year Plan, 156 large projects were newly built, two of which were carbon-related. Similarly, the development of the steel industry relies on carbon materials and products; particularly for electric furnaces smelting alloy steel, graphite electrodes are essential. Aluminum electrolysis consumes 400–450 kg of carbon anode per ton of aluminum produced.
Beyond general civilian industries, carbon products are widely used in aerospace, nuclear, military, and other fields. Carbon fiber composites, with high specific strength and light weight, are used for aircraft, spacecraft, and satellites. Isotropic graphite materials are used for missile nose cones, throat liners, and as neutron moderators, reflectors, and shields in nuclear reactors.
Carbon materials are also widely used in daily life, such as physiological carbon for heart valves, sports equipment like tennis and badminton rackets, and "green tableware" in high-end hotels in developed countries like Japan and South Korea. Carbon products have penetrated industrial, defense, military, and everyday applications.
With industrialization and the global focus on environmental protection, low-carbon economies highlight the importance of carbon in economic development.
Frontiers of Carbon Industry Development
New carbon materials possess a series of excellent properties: high density, high strength, high-temperature resistance, ablation resistance, radiation resistance, low resistivity, good thermal conductivity, low thermal expansion, and good biocompatibility. These are dual-use materials for both civilian and military purposes, and their development is rapid. Beyond the previously mentioned products, there are carbon (graphite) and composite materials, carbon molecular sieves, carbon microspheres, high specific strength and modulus carbon (graphite) structural and functional materials, successfully applied in aerospace, aviation, submarines, nuclear energy, and other industries.
In particular, the discovery of Cm series carbon, carbon nanotubes, and graphene represents a major advance in carbon science, providing broad prospects for carbon chemistry and material research and application, such as high specific strength and modulus structural materials, superconducting and electrode materials, electronic materials and devices, nanomaterials, hydrogen and gas storage materials, catalysts, carbon nanotubes, and graphene materials.
Emerging nanotechnology and carbon nanomaterials will likely drive a technological revolution in the carbon materials industry and the broader industrial field. Carbyne (linear carbon) has long been discovered and awaits industrial application development. Inspired by C60 structures, boron fullerenes have been successfully developed; in the near future, silicon and phosphorus fullerenes are expected, forming a family of spherical-structured substances with extraordinary properties and limitless application potential.
Human development has gradually exhausted terrestrial resources. For sustainable development, countries are exploring the ocean and proposing the "Blue Carbon Plan." "Blue carbon" refers to carbon fixed by marine organisms that can be preserved long-term. The ocean stores enormous carbon, about 50 times the atmospheric carbon stock and 20 times the terrestrial carbon stock. Some inert organic carbon can remain in water for thousands to tens of thousands of years, making the development of the Blue Carbon Plan highly significant. Ocean carbon resources provide a stable raw material base for the sustainable development of the carbon industry.
Feel free to contact us anytime for more information about the Graphite Electrodes market. Our team is dedicated to providing you with in-depth insights and customized assistance based on your needs. Whether you have questions about product specifications, market trends, or pricing, we are here to help.
No related results found








0 Replies