【Graphite Electrode】Internal Heterogeneous Reactions

【Graphite Electrode】Internal Heterogeneous Reactions
Professor Li Wang's team and Professor Xiangming He's team from Tsinghua University published an article in Energy Materials (EM):
Real-time monitoring of current density reveals the evolution of reaction heterogeneity inside graphite electrodes
Yan, Z.; Wang, L.; He, X. Unveiling the pattern and progression of reaction extent heterogeneity inside graphite electrodes through real-time monitoring of current density. Energy Mater. 2025, 5, 500087. http://dx.doi.org/10.20517/energymater.2024.271
The article ingeniously employs multilayer porous copper foils to investigate heterogeneous reactions in multilayer graphite electrodes. The key findings are as follows:
(1) The graphite electrode surface near the separator reacts first, and the reaction gradually propagates inward.
(2) After lithium intercalation at the surface layer becomes saturated, lithium plating begins, and an inflection point appears in the current.
(3) The capacity released by lithium plating at the surface layer compensates for the capacity that has not been utilized in the inner layers of the multilayer electrode, so the apparent total capacity remains unchanged.
(4) Relying solely on total capacity or average voltage cannot truly reflect the internal state of the electrode; localized lithium deposition may already occur before the total SOC is fully reached.
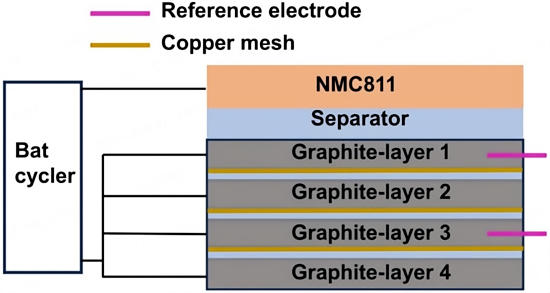
Figure 1. Schematic illustration of the cell structure composed of multilayer graphite electrodes.
The porous copper foil ensures the electrical conductivity of each graphite layer; however, there are significant differences in ion concentration and reaction rate among different graphite electrode layers. This phenomenon is referred to as heterogeneity in the extent of reaction.
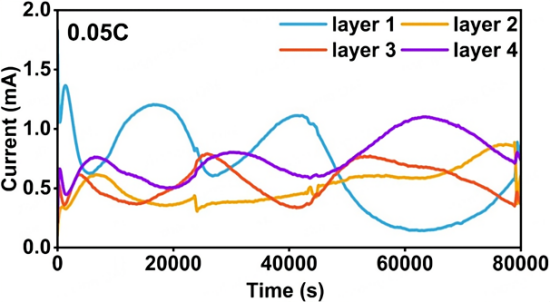
Figure 2. Current density of four-layer graphite electrodes during discharge at 0.05C.
At relatively low cycling rates, the electrode closest to the separator (blue line) reacts first and reacts faster due to higher ion concentration.
Table 1. Capacity of each graphite electrode layer and the total electrode capacity (the theoretical capacity of a single graphite layer is 16.8 mAh; the capacities highlighted in yellow exceed the theoretical value).
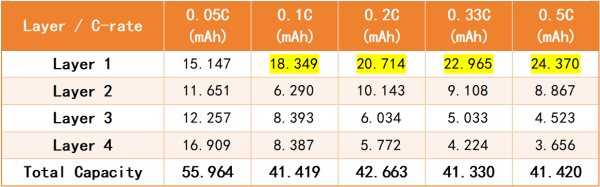
As the charging rate increases, lithium deposition exhibits three characteristics:
(1) Lithium deposition occurs only in the first layer. At 0.1C, 0.2C, 0.33C, and 0.5C, the capacity released by the first layer exceeds its theoretical capacity of 16.8 mAh, while the other layers have not yet reached their theoretical capacities.
(2) As the capacity of the first layer continues to increase, the amount of deposited lithium is proportional to the rate.
(3) Even with lithium plating, the total SOC of the cell does not exceed 1.
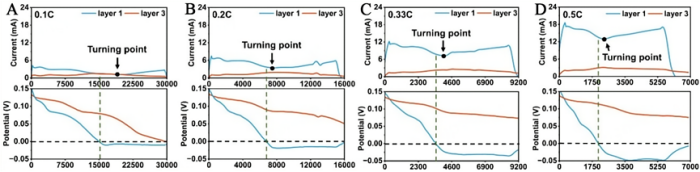
Figure 3. (A–D) Comparison of current density and potential of layer 1 and layer 3 graphite electrodes during discharge at 0.1C, 0.2C, 0.33C, and 0.5C, respectively.
Figure 3 compares the voltages of the first and third graphite layers. At rates of 0.1C, 0.2C, 0.33C, and 0.5C, the voltage of the first layer drops to 0 V, indicating the occurrence of lithium plating in the first layer. The higher the rate, the greater the tendency for lithium plating. For the third layer, at a rate of 0.1C it can still form LiC₆; however, as the rate continues to increase, it can only form LiC₁₂, indicating that a considerable portion of the graphite capacity in the third layer remains underutilized.
Supplementary Figure 1
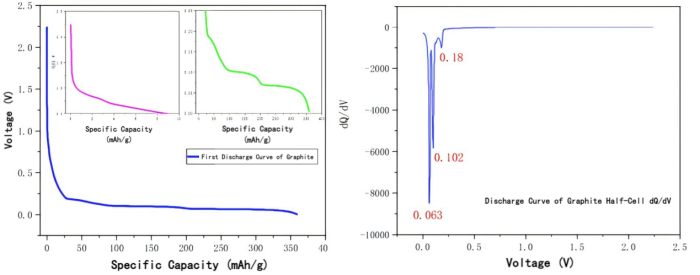
Figure: First lithium intercalation curve of graphite
Supplementary Figure 2
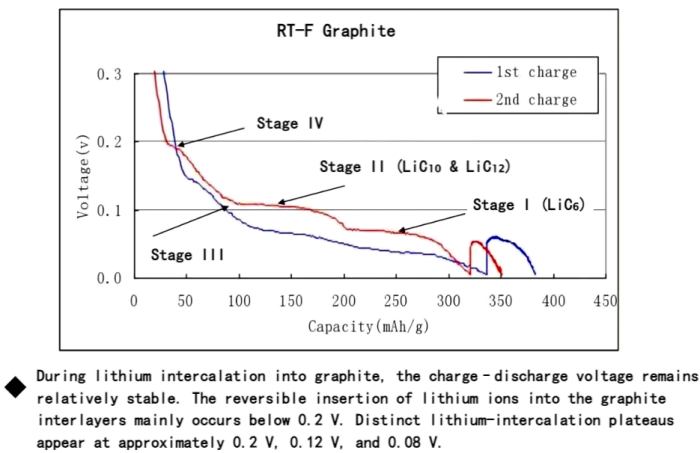
Figure: First and second lithium intercalation curves of graphite
Feel free to contact us anytime for more information about the Graphite Electrodes market. Our team is dedicated to providing you with in-depth insights and customized assistance based on your needs. Whether you have questions about product specifications, market trends, or pricing, we are here to help.
No related results found








0 Replies