【Graphite-Based Anode Materials】"The Four Kings"

The rapid growth of the EV and energy storage industries is boosting demand for high-performance lithium batteries, driving the market for quality petroleum coke and synthetic graphite. The quality and particle size of calcined petroleum coke directly affect synthetic graphite performance, especially in anode production.
【Graphite-Based Anode Materials】"The Four Kings"
Graphite, due to its high electronic conductivity, large lithium-ion diffusion coefficient, layered structure with minimal volume change before and after lithium intercalation, high lithium intercalation capacity (theoretical capacity can reach 372 mA·h/g), and low lithium intercalation potential, became the earliest commercialized lithium-ion battery anode material.
Since the development of lithium-ion batteries, various cathode material systems have been studied, but the graphite-based anode material system has been continuously used. Graphite-based materials have a low lithium intercalation potential and a layered structure suitable for lithium ion insertion/extraction. Current research includes graphitized carbons (natural flake graphite, graphitized mesophase carbon microspheres, artificial graphite, etc.) and non-graphitized carbons (soft carbon, hard carbon, etc.). Under the dual market background of power lithium batteries and consumer lithium batteries, artificial graphite and natural graphite anode materials have become the mainstream in the market, maintaining over 90% of the market share for a long time.
Graphite anodes account for approximately 12%–21% of lithium-ion batteries. Each pure electric vehicle contains about 50 kg of graphite, and each hybrid vehicle also requires about 10 kg of graphite. Even though the current supply exceeds demand, the market demand will continue to be supported for a long period in the future.
Studying the working mechanism of lithium-ion batteries shows that during charging, lithium ions smoothly deintercalate from the LiCoO3 lattice of the cathode, gradually diffuse into the electrolyte, and finally pass through the separator into the graphite anode layer. During this process, to fully ensure charge balance, an equal number of electrons are released from the cathode and flow to the graphite anode through the external circuit, forming a complete loop. During discharge, lithium ions gradually deintercalate from the graphite anode, pass through the electrolyte, and finally reintercalate into the LiCoO3 lattice of the cathode. Electrons are transmitted to the cathode via the external circuit, enabling repeated charge-discharge cycles.
Graphite Anode Charging Process
(1) Artificial Graphite Anode Materials
Artificial graphite is obtained by high-temperature graphitization of petroleum coke, pitch coke, metallurgical coke, mesophase carbon microspheres, needle coke, and other coke materials. Among them, needle coke, as a new type of carbon material, has an excellent graphite microcrystalline structure and needle-like texture, making it an ideal carbon source for lithium-ion battery anode materials. Its advantages include easy graphitization, high conductivity, relatively low price, low ash content, high lithium intercalation capacity, and good reversible lithium intercalation/deintercalation. This ensures requirements for high voltage, high capacity, long cycle life, and high current density. Overall, artificial graphite exhibits better initial Coulombic efficiency, rate performance, and cycling performance than natural graphite and has become the mainstream product in China's power lithium battery anode market in recent years.
However, there are some drawbacks in preparing artificial graphite anode materials from needle coke, such as irreversible reactions with the electrolyte reducing charge-discharge efficiency, solvent co-intercalation causing reversible capacity loss, material volume expansion, and poor cycle performance. These issues hinder the further development of artificial graphite. Additionally, the production process of artificial graphite requires extremely high temperatures (1900–3000°C) and a protective atmosphere, making the production cost far higher than that of natural graphite. Therefore, cost reduction is also an important research topic.
Guo Mingcong et al., using coal-based needle coke as raw material and self-made high-performance coal pitch as a binder, granulated the needle coke to prepare secondary particle artificial graphite anode materials with high energy density and rate performance. The results showed that when 8% pitch binder was added, the granulation process was optimal, forming relatively uniform secondary particle artificial graphite anode materials with a tap density of 1.10 g/cm³. At 0.1C current density, the first charge specific capacity reached 345.7 mAh/g, and the first Coulombic efficiency was 95.6%, higher than secondary particle artificial graphite anodes prepared with other binder amounts. The material also exhibited excellent high-rate charge-discharge capability during rate performance testing.
(2) Natural Graphite Anode Materials
Natural graphite anode materials use flake graphite, microcrystalline graphite, and other raw materials. Due to the anisotropy and small interlayer spacing of flake graphite, directly using it as anode material results in poor cycling and rate performance. Typically, it undergoes balling, purification, carbon coating, and other modification treatments. After flake graphite is spheroidized, its specific surface area is effectively reduced, tap density is increased, and lithium-ion diffusion in the anode material is improved. Therefore, processing natural flake graphite into spherical graphite to make anode materials can greatly enhance battery performance.
The spheroidization process of natural flake graphite mainly includes grinding and jet milling, with similar mechanisms. High-quality high-carbon flake graphite is used as raw material, and mechanical forces in the spheroidization machine—collision, shear, and friction—act on the particles, breaking large graphite particles and allowing small particles to agglomerate, forming relatively uniform spherical graphite. After full spheroidization, the yield is usually only 40%–50%, with a high proportion of fine powder waste, most of which can be used for low-value products such as lubricants and sealing materials.
SEM images of flake graphite and spherical graphite
anode materials
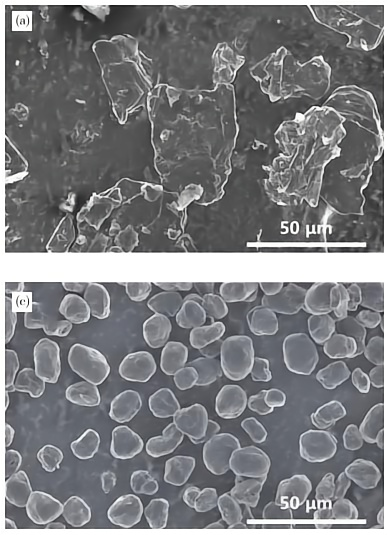
Dong Huazhong et al. used graphite concentrates from Jixi, Inner Mongolia, and Luobei, each with a fixed carbon content of approximately 95%, as raw materials. They sequentially performed spheroidization shaping and chemical purification to prepare high-purity spherical graphite and tested its electrochemical performance. The experiments showed that the first charge-discharge efficiency of graphite anode materials from all three regions was greater than 90%, and the first discharge capacity exceeded 360 mAh/g, making them ideal raw materials for preparing natural graphite anode materials.
However, natural graphite anode materials mainly use flake graphite as raw material but have several drawbacks:
① Flake graphite has a small specific surface area, leading to low initial charge-discharge efficiency.
② Its anisotropy is unfavorable for Li⁺ diffusion within the material.
③ Layer cracks occur due to lithium intercalation/deintercalation, increasing Li⁺ diffusion resistance.
(3) Amorphous Carbon
Amorphous carbon materials consist of amorphous carbon and graphite microcrystals, containing numerous pores that can serve as lithium storage sites during charge-discharge, increasing specific capacity. Therefore, their theoretical capacity is higher than graphite’s 372 mAh/g. Amorphous carbon is divided into soft carbon and hard carbon.
Soft carbon materials contain many graphitic microcrystalline regions arranged quasi-parallel and can be graphitized at high temperatures (above 2500°C). Soft carbon is generally obtained by heat treatment of coke below 1500°C and has a large interlayer spacing (>34 nm), short-range order, and long-range disorder. Therefore, it exhibits low specific capacity (<300 mAh/g), low initial Coulombic efficiency (<80%), and low tap density. However, it excels in rate performance and long-term cycling performance, giving it significant potential in commercial applications. Research indicates that combining soft carbon with graphite can complement their advantages and disadvantages, enhancing battery rate performance and cycling stability.
Hard carbon refers to carbon materials that are difficult to graphitize even above 3000°C. Its structure contains many curved graphite layers (pseudo-graphite regions), with interlayer spacing larger than graphite, forming 2–6 layered structures. In addition to graphite layers, hard carbon contains numerous micropores, which can adsorb lithium ions during charge-discharge. However, these micropores also reduce initial Coulombic efficiency.
(4) Graphene
Besides the above materials, other commonly used carbon-graphite anode materials for lithium-ion batteries include graphene and carbon fibers. Zhang Tiange et al. found that compared with traditional anode materials, two-dimensional Fe3O4/graphene composites exhibit excellent anti-polarization and conductivity, with good electrochemical performance. Compared with two-dimensional Fe3O4/graphene composites, three-dimensional graphene network Fe3O4/graphene composites show slower specific capacity decay, better cycling stability, superior electrochemical performance, and great application potential.
Outlook
In the current lithium-ion battery material system, next-generation anode materials, such as silicon-carbon and lithium metal, have immature industrial application technologies, high costs, and matching electrolyte and binder systems that require further development, making it difficult to replace graphite as the mainstream in the short term. Graphite anode materials will remain the absolute market mainstream, although the industry structure may undergo significant changes. Under the "carbon peak, carbon neutrality" strategic goals and energy consumption "dual control" policies, natural graphite, with advantages such as no graphitization process, low cost, and stable industrial and supply chains, will see increased penetration in various applications.
Moreover, to address the challenges of traditional graphite anodes not meeting increasingly high-performance requirements, many domestic and international scholars have actively explored modification technologies with fruitful results. Effective strategies to improve graphite anode rate performance include reducing graphite particle size, doping, surface coating, co-intercalating expanded graphite layers, and designing new electrolytes or additives. To increase graphite anode capacity, methods such as doping and composite formation are used. For cycling stability and safety, surface coatings and electrolyte additives are employed to maintain graphite layer structure stability during charge-discharge and to build a stable SEI film.
Feel free to contact us anytime for more information about the Anode Material market. Our team is dedicated to providing you with in-depth insights and customized assistance based on your needs. Whether you have questions about product specifications, market trends, or pricing, we are here to help.
No related results found








0 Replies